One year after the declaration of the Covid-19 pandemic, the figures provided by the Center for Systems Science and Engineering (CSSE) of Johns Hopkins University report 124 million infected people and 2.7 million deaths in the world, because of this infection.
The speed of contagion, the spread of the disease throughout the planet, the saturation of hospitals and the economic and social impacts of the health situation, motivated a rapid action by various groups of scientists, in search of a solution.
In April 2020, the World Health Organization (WHO) created the Access to Tools Accelerator against Covid-19 (ACT Accelerator), as a global collaborative initiative for the development, production and equitable access to tests, treatments and vaccines against this disease.
Within the ACT Accelerator, the platform dedicated to the development of vaccines has been called Covax and is co-directed by the Coalition for the Promotion of Innovations for Epidemic Preparedness (CEPI), the Alliance for Vaccines, Unicef, vaccine manufacturers from various countries, the World Bank and WHO, among other entities.
Covax works with different governments and manufacturing laboratories to ensure that Covid-19 vaccines are available to all countries. Therefore, it has been at the forefront of the validation of the different investigations that are being carried out to develop vaccines.
By the end of 2020, the international body had counted more than 200 experimental vaccines, with 61 products that have already advanced to the human trial phase. On December 31, 2020, the Cominarty vaccine from Pfizer / BioNTech was the first to be validated for use in an emergency situation, by the WHO, thus authorizing its inoculation in humans.
Until March 2021 the WHO has also authorized the two versions of the AstraZenaca / Oxford vaccine – SKBio and the Serum Institute of India; Modern and Johnson & Johnson’s Janssen Vaccine. There are several vaccines against Covid-19 approved for use by the health authorities of various countries. Currently there are 11 vaccines that are already being inoculated worldwide.
The Milken Institute Vaccine Tracker: COVID-19 Vaccine Tracker, https://www.covid-19vaccinetracker.org/ allows you to check the type and phase of each product, the nations where its inoculation has been authorized, its composition, among other important data.
Different mechanisms to design vaccines against Covid-19
Since December 2020, the vaccination against Covid-19 began, with the different vaccines that have been authorized. The variety of products approved is due to the number of experimental vaccines that must prove their safety and efficacy before being approved for use.
According to the WHO, “7 out of 100 vaccines that are analyzed in laboratories and tested in experimental animals are considered good enough to move to the phase of conducting clinical trials with humans. Of all the vaccines that reach the clinical trial phase, only 1 in 5 proves to be of real utility. Developing a large number of different vaccines increases the chances that there will be one or more vaccines that will prove useful and will prove to be safe and effective for the demographic groups that you intend to prioritize. ” (The different types of vaccines that exist )
Currently, there are three main types of COVID-19 vaccines that are licensed, or are in the stage of large-scale clinical trials. Their differentiation is due to the method with which they are designed and we describe them below, with information from the United States Centers for Disease Control (CDC) .
The mRNA (Messenger RNA) vaccines contain material from the virus that causes COVID-19, when inoculated it triggers an immune response within the body to produce antibodies, which will protect the body if the real virus enters the body.
Protein subunit vaccines instead of using the whole virus, use only the proteins or smaller parts, such as peptides, that can be obtained by recombinant biotechnology in the laboratory.
Vector or adenovirus vaccines contain a modified version of a virus other than the one that causes COVID-19. Inside the envelope of the modified virus, there is material from the agent that causes COVID-19, known as a “viral vector.” Once the viral vector enters the cells, the genetic material instructs the cells to produce a protein, unique to COVID-19, which elicits an immune response to defend the body if exposed to the coronavirus.
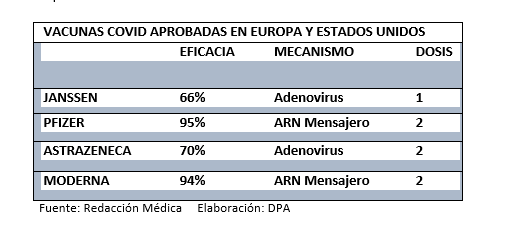
It is important to note that comparing the effectiveness of vaccines can be difficult because each one had different circumstances and development contexts, as shown in this Vox report:
SOURCES:
WHO Emergency Use Listing Procedure: https://www.who.int/publications/m/item/emergency-use-listing-procedure
Frequently Asked Questions about COVID-19 Experimental Vaccines and Access Mechanisms. Technical Document, PAHO:
COVAX: Collaboration for Global Equitable Access to COVID-19 Vaccines, WHO:
https://www.who.int/es/initiatives/act-accelerator/covax
The different types of vaccines that exist, WHO
https://www.who.int/es/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained
Vaccine Tracker, Milken Institute:
https://www.covid-19vaccinetracker.org/
Johns Hopkins University Center for Systems Science and Engineering (CSSE) : https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
Interviews:
Dr. Mary Vallecillo, a researcher in the Department of Chemistry and Biochemistry at Brigham Young University.
Dr. Diana Vanegas, pediatrician, teacher and director of the Master of Sexuality at the University of Cuenca.
Photography: Prasesh Shiwakoti (Lomash)



